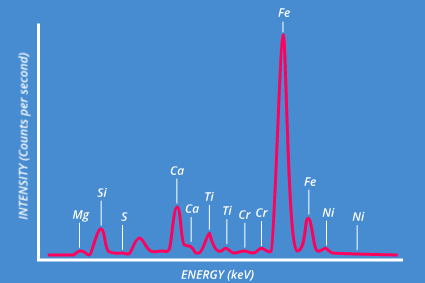
XRF (and particularly EDXRF) is ideally suited for very fast qualitative elemental analysis. Typically all elements from sodium through to uranium can be detected simultaneously, with good quality spectra obtained in seconds/minutes.
Band assignment for an XRF spectrum is usually easy, since each element peak occurs at a known fixed position - however, overlapping bands can cause confusion, but modern software will take this into account for peak labeling.
Similarly, certain artifact peaks may be present in the spectrum, including Rayleigh and Compton scattered characteristic lines from the X-ray generator, peaks caused by X-ray diffraction, and sum/escape peaks. Knowledge of these is necessary to avoid incorrect interpretation of results.
In general, concentrations from 100% down to sub-ppm are detectable with XRF, the lower limit depending on the particular instrument configuration.
XRF is a quantitative technique – the peak height for any element is directly related to the concentration of that element within the sampling volume. However, care must be taken, because two or more elements can interact with each other, resulting in skewed results. For example, chlorine atoms strongly absorb fluorescent X-rays (Ka) from lead – thus, if chlorine is present, the observed lead signal will be much less intense than expected for a particular concentration.
Quantitative analysis is usually carried out using two main methods, both of which are usually fully incorporated within typical instrument software.
Comprehensive software algorithms based on theoretical X-ray beam intensity, detector solid angle, matrix effects (element-element interactions), band overlap and spectral backgrounds are used to calculate element concentrations based on the observed peak intensities. FPM provides a very fast, robust quantification method, which will work well for various matrices and experimental conditions (eg, voltage, current, beam size etc).
Calibration standards with accurately known element concentrations are used to generate calibration curves (XRF peak intensity versus concentration). These curves are then used to calculate concentrations from observed spectra. This method works extremely well, and would be recommended for best accuracy. However, matrix effects are not taken into account, so calibration standards can only be used for analysis of samples with similar (if not identical) matrices. As an example, a calibration curve generated with a set of metal alloys will most likely yield incorrect values when analyzing mineralogical samples.
It is known that X-rays will penetrate some way into a material. For XRF analysis, the important question is from what depth within the sample does the spectrum arise. Unfortunately this is not a simple question, as there are many factors involved.
The two main points to consider are (a) the depth of penetration of the primary X-ray beam into the sample, and (b) the escape depth from which fluorescent X-rays can be detected. Both of these are directly linked to the energy of the X-rays - the higher the X-ray energy the deeper the X-ray penetrates. In general, it is fair to assume that X-rays will penetrate a few micrometers down to several millimeters, depending on the sample matrix. At best fluorescent X-rays will be detectable from a few millimeters within the sample, but in many situations this could be reduced to a few micrometers or less.
The primary X-rays should be considered in two parts, both of which are effected by the voltage setting of the X-ray generator.
First of all, the characteristic X-rays from the anode target material are at a fixed energy. If the generator voltage is sufficient to excite multiple lines (eg, K and L) then both high energy (K) and low energy (L) X-rays will be incident on the sample. Usually the K lines will be more intense, and so these will dominate in considerations about penetration. If however, the voltage is reduced to such an extent that the higher energy X-rays are no longer excited, then the characteristic X-rays will be low energy L lines only - as a result the expected penetration will be greatly reduced.
Secondly, the bremsstrahlung (or continuum) X-rays must be considered. As their name suggests, these X-rays have a continuous energy range (up to a maximum equal to the accelerating voltage of the generator. The continuum spectrum is most intense towards the higher energy cut off - by reducing the voltage it is possible to reduce this "average energy" of the continuum, and thus reduce penetration.
The ability of fluorescent X-rays to penetrate through and escape from the sample itself depends again on their energy, which directly relates to which elements are being detected. The lighter elements (eg, Na, Mg, Al, Si) have very low energy X-rays, and thus will be difficult to detect even at relatively small depths within the sample. Heavier elements (eg, Cu, Ag, Au) have much more energetic X-rays which will be able to pass through large distances within the sample.
Clearly the sample composition itself is also an important factor. The higher the concentration of heavier elements which absorb strongly, the more reduced the chance of X-rays escaping from deep within the sample.
To summarize, heavy elements (ie, energetic fluorescent X-rays) will be detectable relatively deep within a sample matrix primarily comprised of light elements (ie, low absorption). Light elements (ie, low energy fluorescent X-rays) will be detectable only at the surface of a sample matrix comprised of heavy elements (ie, strong absorption).
A typical micro-XRF instrument such as the XGT systems includes a high precision motorized sample stage, which allows the sample to be very accurately positioned beneath the X-ray beam. Color video cameras allows the sample to be visualized by the operator. Typical instrument software will enable fast sample positioning by seamlessly linking camera images with sample movement.
In addition to manually aligning the sample so that a specific feature can be discretely analyzed, it is also possible to store in the software a list of positions for analysis. Once these have all been selected and the measurement conditions chosen, the motorized stage will move to each position in turn, acquiring a spectrum at each before moving to the next.
In this manner, different visual features across the sample can be automatically analyzed with ease, or indeed, multiple samples laid out on the sample stage can be analyzed in turn. In this manner, time consuming repeat measurements are done automatically, leaving the instrument operator free for other activities.
Do you have any questions or requests? Use this form to contact our specialists.