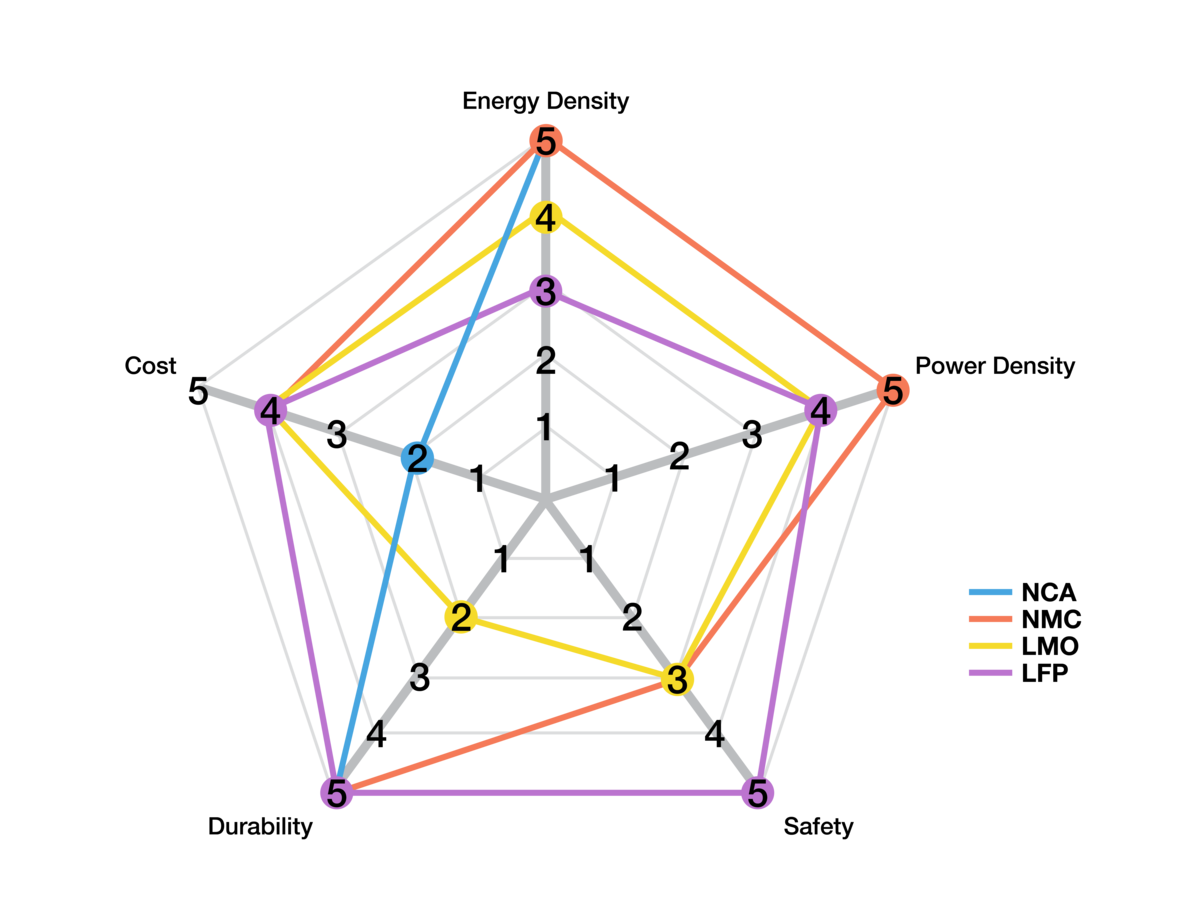Lithium-ion (Li-ion) battery cells are widely used in various industries such as automotive, consumer electronics, and stationary storage, leading to the development of diverse battery cells to meet different needs. This diversity necessitates specific testing requirements and an understanding of each type's unique behavior.
This article discusses the current and expected future variations in Li-ion cells and their testing implications, providing essential knowledge for designing laboratories to test a wide array of current and future Li-ion cells.
A 'Li-ion' cell uses lithium ions' intercalation for energy storage and transfer.
When charging, lithium ions move from the cathode to the anode through the electrolyte, and vice versa during discharge, with electrons balancing the charge via an external circuit.
A separator allows ion but not electron transfer, forcing electrons through the external circuit. Current collectors connect the electrodes to this circuit.
For enhanced energy storage, larger cells repeat this component combination, featuring multiple layers over large areas forming a ‘jelly roll’.
Li-ion cells are preferred in many markets for their high energy density, power capability, and longevity. Their design varies to meet the demands of different applications. Common design criteria for energy storage applications include:
The importance of these criteria varies by application, with automotive placing a high value on energy density for vehicle efficiency and range.
Chemistry variations in the anode, cathode, and electrolyte, along with unique additives, coatings, and intellectual property, enable manufacturers to enhance electrode and electrolyte performance.
Cell designs, including cylindrical, prismatic, pouch, and button formats, also vary, each offering distinct advantages and drawbacks.
Both chemistry and design are finely adjusted to optimize performance characteristics.
An overview of Li-ion cell variations is shown in Figure 2.
Figure 2: Range of Lithium-Ion battery cell designs and chemistries.
Figure 3: Cathode chemistry variations.
The high potential electrode (cathode) significantly influences cell characteristics. Key cathode materials like Nickel-Cobalt-Aluminum (NCA) and Nickel-Cobalt-Manganese (NCM) are preferred in applications valuing energy storage and range. They combine high operating potential with a balance of energy, power, durability, and cost. Lithium-Iron-Phosphate (LFP) cathodes are suitable for fast charging, safety, and longevity. They traditionally have lower energy density but recent advancements are enhancing their competitiveness.
Relative cathode attributes are shown in Figure 3.
The choice of cathode material impacts Li-ion cell behavior in several ways:
Figure 4: Anode chemistry variations.
The anode, acting as the low potential component opposite the cathode, typically includes primarily graphite for its low operating potential, high energy density, and affordability. Despite its advantages, graphite facilitates a range of Li-ion cell ageing mechanisms. Lithium-Titanate-Oxide (LTO) offers an alternative with longer lifespans at the cost of lower cell voltage and energy density due to its higher anode potential.
Silicon is emerging as a promising option or addition to graphite, providing higher energy density. Silicon however faces challenges with volume changes during lithium intercalation, which can lead to rapid degradation if not managed properly.
Relative anode attributes are shown in Figure 4.
The choice of anode material also influences cell behavior:
Electrolytes operate within a specific voltage 'stability range,' requiring compatibility with electrode materials. They significantly affect degradation and safety, influenced by reactions with electrodes and flammability. LiPF6 is widely used in Li-ion cells despite its stability issues and flammability, which can cause thermal runaway by generating excessive heat rapidly. Many manufacturers enhance LiPF6's stability and reduce ageing through proprietary additives.
The choice of electrolyte gives the following considerations:
Figure 5: Illustration of the main cell formats: Cylindrical, prismatic, and pouch.*
Cell materials come in various formats, predominantly cylindrical, prismatic, and pouch (Figure 5*). Button cells are an option typically reserved used for small power applications e.g. hearing aids.
Cell format and size can influence behavior in multiple ways:
*Schröder, R., Glodde, A., Aydemir, M., & Bach, G. (2015). Process to Increase the Output of Z-Folded Separators for the Manufacturing of Lithium-Ion Batteries. Applied Mechanics and Materials, 794, 19–26. https://doi.org/10.4028/www.scientific.net/amm.794.19
Li-ion technology is advancing with improvements in current technologies, new electrode and electrolyte materials, and innovative approaches beyond lithium intercalation. Key emerging trends include:
In this section, some of the key differences in Li-ion battery cells will be captured to understand how it could affect aspects of a battery cell laboratory.
Figure 6: Components of a battery cell testing laboratory.
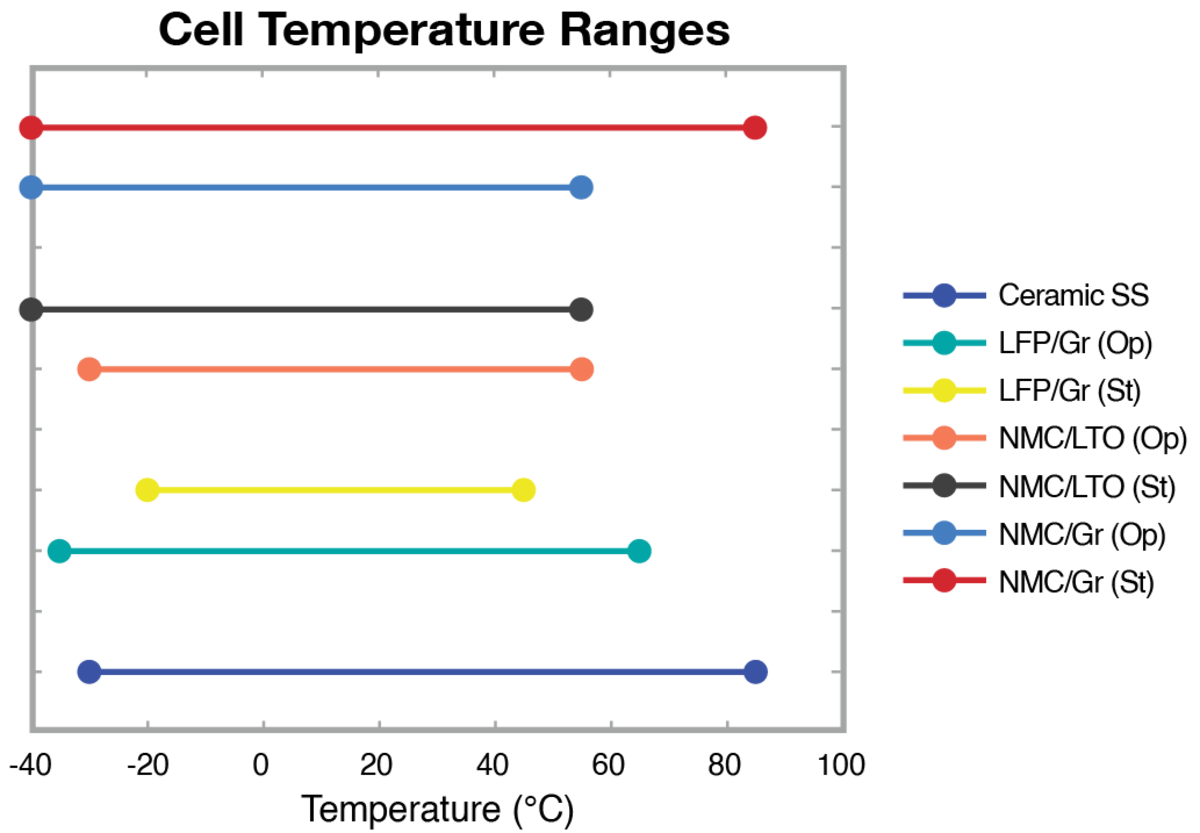
The operating temperature range of Li-ion cells depends on their chemistry, affecting the choice of thermal chambers and chillers needed to test all cells for a specific application.
Figure 7: Selection of Li-ion battery cell temperature ranges.
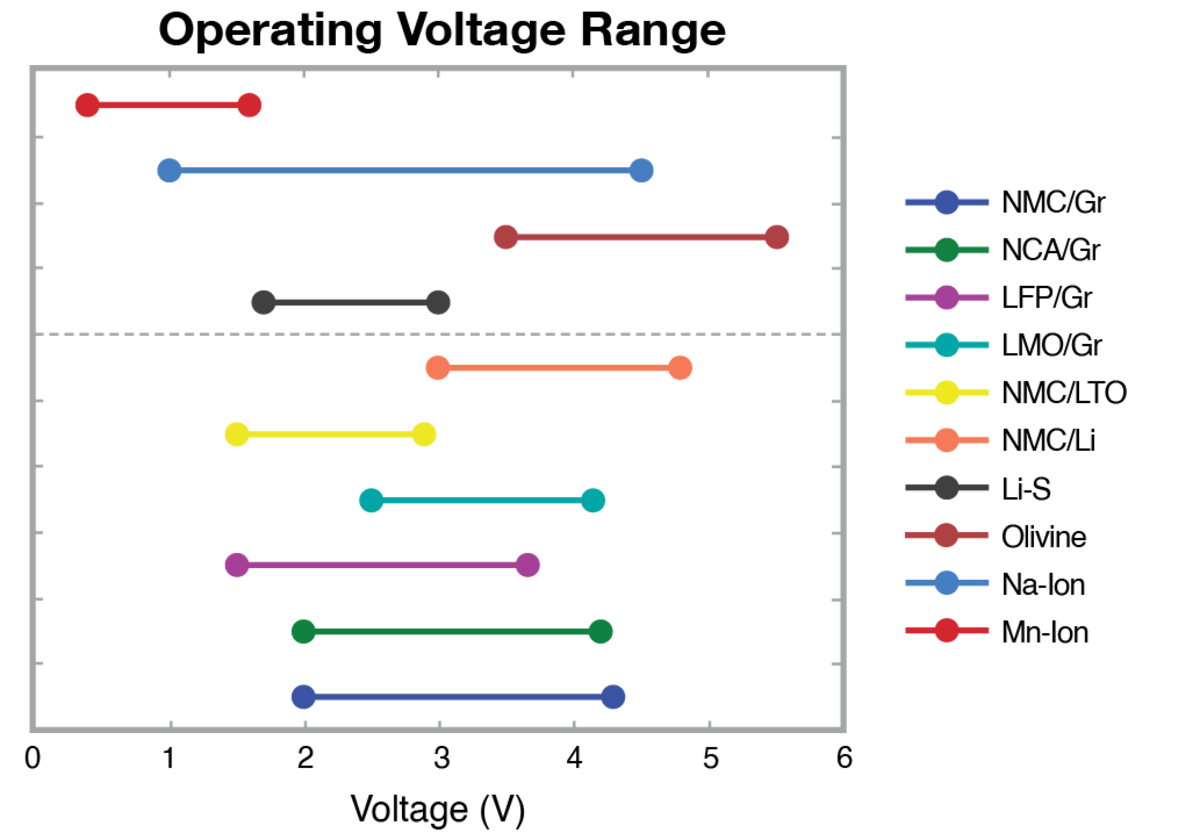
The operational voltage range of Li-ion cells differs with anode and cathode chemistries, influencing the choice of cell cyclers. It's vital to select a cycler with a suitable voltage range for improved accuracy and resolution while ensuring it can accommodate all potential cell choices for an application.
Figure 8: Selection of Li-ion battery cell voltage ranges.
The Open Circuit Voltage (OCV) of a Li-ion cell, influenced by its chemistry, indicates the rest potentials difference between the cathode and anode at a certain State-of-Charge (SoC). OCV typically rises nonlinearly with SoC increase.
This increase varies with chemistry, especially in its rate of change with SoC. LFP cathodes and LTO anodes produce flatter OCV-SoC curves compared to NMC cathodes and graphite anodes. Accurately capturing the curves for these chemistries demands high voltage resolution and precision.
Future technologies like Li-S and Sodium-Ion battery cells, with minimal OCV shifts at certain SoCs, will similarly require precise voltage measurements.
Li-ion cells vary significantly in formats and size which influences their heat generation and dissipation. All cells produce heat due to resistance to electrical energy transfer, following Ohm's law. Higher resistance and current-capable cells generate more heat. This aspect is crucial for defining thermal chamber capabilities.
For larger or high-current cells active cooling may be required. This is typically done through chillers for fluid circulation and thermal connection during testing. Larger cells' volume-to-surface area ratio can cause internal heat buildup. This poses challenges for effective cooling and temperature estimation. Terminal cooling sometimes offering a more effective cooling method. Cell format also determines the testing fixture for optimal thermal interaction with the cooling system.
Additionally, depending on the cell's format and chemistry, pressure control and external housing might be necessary, especially for pouch cells to prevent swelling and for chemistries like those with solid electrolytes or silicon anodes that demand specific pressures or volume changes. This can guiding test setup design and necessitate pressure measurement.
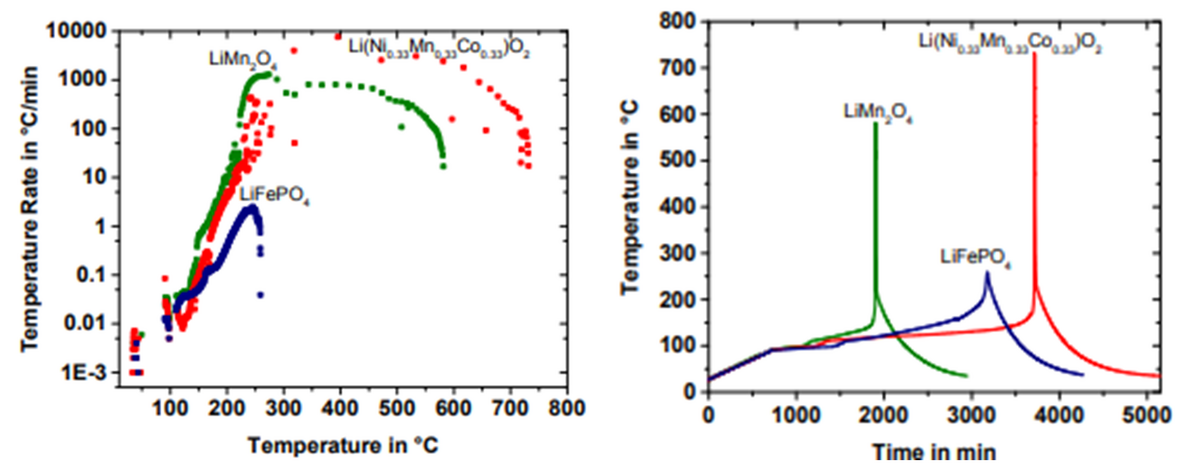
All Li-ion battery cells can experience thermal runaway, with the likelihood, temperature threshold, peak temperatures, and gas emissions varying by chemistry and design. Larger cells, storing more thermal energy, pose a greater risk and emit more gas during runaway. These considerations are crucial for lab safety and the choice of test chambers, with larger cells necessitating chambers equipped for enhanced safety and better heat and emission management.
Figure 10: Thermal runaway behavior with chemistry***
Designing a lab optimum for the target testing requirements and application while simultaneously versatile enough for the range of possible battery cells is challenging. To design effectively, a large range of data, calculations and consultancy is required.
We have experience in testing, modeling and understanding Li-ion battery cells, as well as extensive experience in lab design. By combining these two areas of expertise, we can assist in developing a lab suitable for your requirements.
Li-ion cell ageing: Considering Li-Ion Battery Cell Ageing in Automotive Conditions
EIS for Solid State Batteries: Electrochemical Impedance Spectroscopy for All-Solid-State Batteries: Theory, Methods and Future Outlook
Thermal management: Why Managing Battery Temperature is Vital
Do you have any questions or requests? Use this form to contact our specialists.